Siegel Lab - Research Program in Cancer Metastasis
Peter Siegel Ph.D., Principal Investigator
RESEARCH OVERVIEW: The Metastatic Cascade
Metastatic tumor cells must overcome numerous barriers that prevent their dissemination to distant organs and tissues. Traditionally, these steps have been broken down to include local invasion through the basement membrane, migration and intravasation into the lymphatic or hematogenous systems, survival during transit in the circulation, evasion of host anti-tumor immunity, adhesion/extravasation at the target site and reestablishment of a growing tumor mass in a foreign microenvironment. Communication between the tumor and its microenvironment is likely to be the single most important determinant for organ-specific metastasis. Thus, both tumor intrinsic and microenvironmental factors play important roles in controlling the metastatic behavior of cancer cells.
RESEARCH PROGRAM
We have established a comprehensive research program to study the molecular mechanisms that control the metastatic process in solid cancers. Current research ongoing in my laboratory encompasses several broad research areas, which are briefly outlined below.
I. Molecular and Cellular Mechanisms that Control Cancer Cell Migration and Invasion
Cell migration and invasion are two fundamental cellular processes that contribute to the metastatic ability of cancer cells. Focal adhesions are cellular structures that form on the ventral surface of cancer cells, which links the extracellular matrix to the cell cytoskeleton. Control of adhesion assembly and disassembly is critical for the movement of cancer cells. Invadopodia are distinct cellular structures that form on the ventral surface of cancer cells, which are responsible for the local degradation of extracellular matrix components. Invadopodia allow cancer cells to invade through tissues. We investigate the role that biochemical (TGFb) and biophysical cues (tissue/matrix stiffness) play in modulating the function of cellular adhesions and invadopodia, which impacts the metastatic ability of cancer cells.
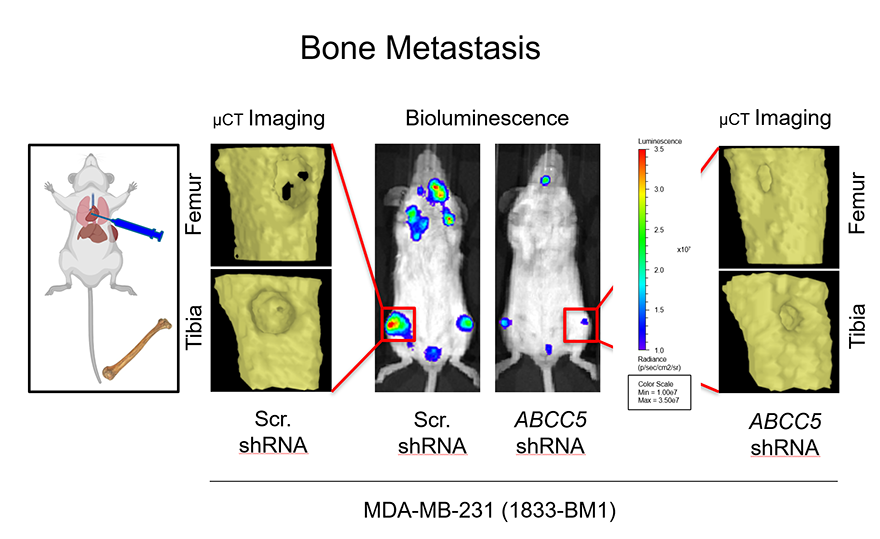
II. Mediators of General and Organ-Specific Breast Cancer Metastasis
We have used gene expression profiling approaches to identify genes whose expression is elevated in cancer cells that are metastatic to distinct organs/tissues. While our initial focus has been in the context of breast cancer, we investigate the role of mediators we identify in the context of other solid cancer types. These studies have uncovered cell surface proteins (GPNMB, Claudin-2), secreted factors (CCN3), adaptor/scaffold molecules (ShcA, Lpp, Afadin) and RNA binding proteins (CIRBP). The lab clinically validates the importance of these mediators in patient-derived samples (primary tumors and metastases). We then establish a functional role for these candidates in modulating tumor growth and metastasis using syngeneic mouse models, transgenic mouse models and patient-derived xenograft models. Finally, we try to elucidate the molecular and cellular mechanisms through which these factors regulate tumor growth and metastasis.
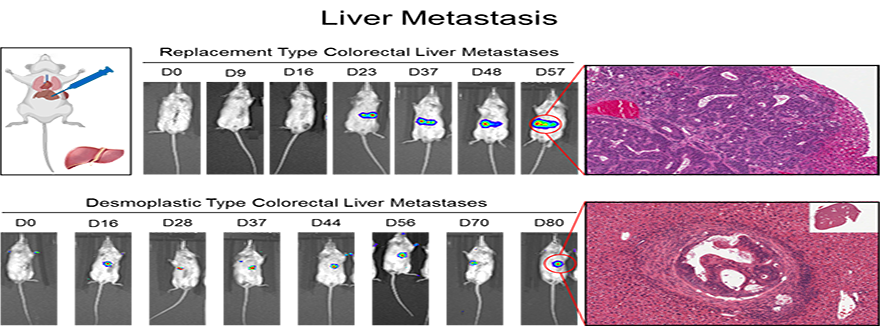
III. Metabolic Adaptation/Plasticity Promotes Metastatic Fitness
Cancer cells must continually alter their cellular metabolism to cope with changing microenvironments in both the primary tumor and distant metastases. Our work has shown that specific metabolic strategies (glycolytic metabolism, oxidative phosphorylation) enable cancer cells to grow some sites (lung, bone), versus others (liver). We have also shown that cancer cells that exhibit the ability to shift between different metabolic strategies are the cells that are the most metastatic and therapy resistant. Currently, we are examining how distinct metastatic or primary tumor microenvironments influence metabolic wiring in cancer cells.
IV. Development of Experimental Therapeutics and Combination Therapies to Treat Metastatic cancers
Our ultimate goal is to take the information we gain from the projects described above to identify candidate targets against which novel therapeutic agents can be devised to better clinically manage metastatic cancer. We have several ongoing projects in which therapeutic agents are being generated and tested against targets that emerge from our studies.